Dracen Pharmaceuticals Announces the Initiation of
First-in-human Study of DRP-104 in Adult Patients With Advanced Solid Tumors
JANuary 29, 2026
A Phase 2 Study is open for accrual: "DRP-104, a Glutamine Antagonist, in Patients with NFE2L2/KEAP1-altered Non-Small Cell Lung Cancer following standard of care treatment with chemotherapy and immunotherapy"
Please refer to www.clinicaltrials.gov to (Identifier: NCT07249372)
for additional clinical trial information.
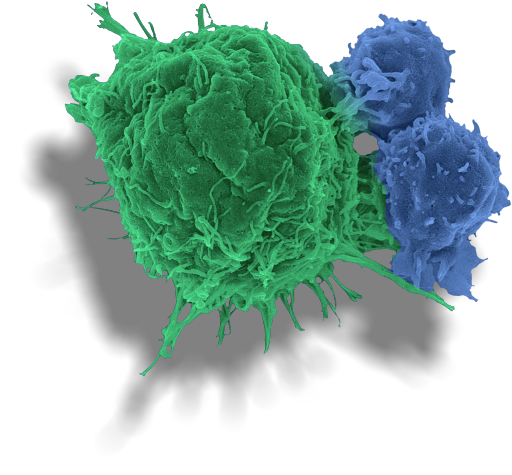




Dracen is a private pharmaceutical company leveraging immuno-metabolism to create a potential new treatment paradigm with broad application for many cancer types. Our goal in treating cancers is to directly shrink tumors and remodel the tumor microenvironment to make it more conducive to immuno-oncology approaches, to gain greater disease control, increase anti-tumor responses and extend patient survival in areas of unmet need. This includes cancers that possess genetic mutations which may make them highly susceptible to our therapies and those able to evade available immuno-oncology and other standard-of-care approaches.
Our lead candidate, sirpiglenastat (DRP-104), is unique among glutamine inhibitor approaches as it irreversibly inhibits all known enzymes involved in glutamine metabolism resulting in:



A founding executive of Dracen, Dr. Ragab was most recently the Vice President, Search and Evaluation, Oncology at Bristol-Myers Squibb where he was involved in many major immuno-oncology deals. Prior to that, he was Global Partnering Head, Oncology at Hoffman-La Roche (member of the Roche Group) and prior to that global commercial leader for oncology at Pfizer. He also held various positions at Ortho Biotech and Schering-Plough (now Merck & Co.). He holds a Master Degree in Radiation Oncology from Cairo University School of Medicine and a Bachelor of Medicine and Surgery degree from Cairo University School of Medicine.

Mr. Estok brings 30+ years of experience in development and commercialization of pharmaceuticals to Dracen. Previously, he was the founding President/CEO of Tragara Pharmaceuticals, founding President/CEO of Cabrellis Pharmaceuticals where he led the organization’s development of amrubicin prior to its acquisition by Pharmion, and served as Chief Commercial Officer of Conforma Therapeutics whose HSP90 franchise was acquired by Biogen-Idec. A 19-year veteran of Schering-Plough, Mr. Estok held a number of positions with progressing responsibility in R&D (biochemistry and oncology operations management), in both US and global marketing, European operations, sales and commercial development. His most recent position was Vice President, US Marketing and Commercial Development, Oncology. Mr. Estok has led multidisciplinary R&D, business development, and commercial teams and is a recognized leader in the commercialization of oncology therapeutics and the formation/growth of new divisions and companies. Mr. Estok graduated from Virginia Tech with a BS degree in Biochemistry and was awarded an MBA from Fairleigh Dickinson University. He is currently a Board Director at Adastra Pharmaceuticals.


Mr. Estok brings 30+ years of experience in development and commercialization of pharmaceuticals to Dracen. Previously, he was the founding President/CEO of Tragara Pharmaceuticals, founding President/CEO of Cabrellis Pharmaceuticals where he led the organization’s development of amrubicin prior to its acquisition by Pharmion, and served as Chief Commercial Officer of Conforma Therapeutics whose HSP90 franchise was acquired by Biogen-Idec. A 19-year veteran of Schering-Plough, Mr. Estok held a number of positions with progressing responsibility in R&D (biochemistry and oncology operations management), in both US and global marketing, European operations, sales and commercial development. His most recent position was Vice President, US Marketing and Commercial Development, Oncology. Mr. Estok has led multidisciplinary R&D, business development, and commercial teams and is a recognized leader in the commercialization of oncology therapeutics and the formation/growth of new divisions and companies. Mr. Estok graduated from Virginia Tech with a BS degree in Biochemistry and was awarded an MBA from Fairleigh Dickinson University. He is currently a Board Director at Adastra Pharmaceuticals.


Dr. Wild joined Dracen Pharmaceuticals in 2017 as one of the founding executives and Chief Scientific Officer (CSO). He is responsible for all discovery, preclinical development and translational research efforts of Dracen’s therapeutic pipeline and leads scientific strategy. Dr. Wild is a seasoned R&D executive with 20+ years of broad experience in drug discovery and development, spanning both small molecules and biologics. Throughout his career, Dr. Wild has contributed to the advancement of numerous clinical development candidates, including four FDA-approved oncology therapeutics (sunitinib, cetuximab, dasatinib and erlotinib). Before Dracen Pharmaceuticals, Dr. Wild was an independent consultant to biotech, pharma and venture capital companies. Prior to that, he served in various leadership roles including Chief Scientific Officer and Senior Vice President of Research at Ignyta, Inc. (now member of the Roche Group), Chief Scientific Officer Oncology Research at Eli Lilly & Company, Senior Director at OSI Pharmaceuticals (now a wholly owned subsidiary of Astellas Pharma), Senior Research Investigator at Bristol-Myers Squibb and Scientist at SUGEN, Inc. (acquired by Pfizer). Dr. Wild is a native of Germany and received his BS in Biochemistry and PhD in Pharmacology from the University of Minnesota, Minneapolis.


Mr. Bilski has more than 20 years of experience in accounting, finance, strategic planning, and executive management for public and private companies. Mr. Bilski was the Chief Financial Officer of Tragara Pharmaceuticals, Inc. from 2007-2018. Prior to Tragara, Mr. Bilski was the Director of Finance at Del Mar Database (DMD), a software and services provider for mortgage lenders. Mr. Bilski played a key role in leading DMD through a turnaround and rapid expansion and subsequent acquisition by Fiserv, Inc. Prior to DMD, Mr. Bilski spent five years at Arthur Andersen LLP in Washington, D.C., where he managed financial statement audits and due diligence engagements for various public and private companies. Mr. Bilski also spent two years with a consulting firm, where he worked exclusively with early-stage technology firms. Mr. Bilski graduated from The College of William and Mary with a BBA degree in Accounting.


A founding executive of Dracen, Dr. Ragab was most recently the Vice President, Search and Evaluation, Oncology at Bristol-Myers Squibb where he was involved in many major immuno-oncology deals. Prior to that, he was Global Partnering Head, Oncology at Hoffman-La Roche (member of the Roche Group) and prior to that global commercial leader for oncology at Pfizer. He also held various positions at Ortho Biotech and Schering-Plough (now Merck & Co.). He holds a Master Degree in Radiation Oncology from Cairo University School of Medicine and a Bachelor of Medicine and Surgery degree from Cairo University School of Medicine.







Barbara Slusher is Professor of Neurology (primary), Psychiatry, Neuroscience, Medicine and Oncology at Johns Hopkins School of Medicine and the Director of the Johns Hopkins Drug Discovery program (https://drugdiscovery.jhu.edu/). Before joining Johns Hopkins, she spent 18 years in the pharmaceutical industry, including several years at the level of Senior Vice President of Research and Translational Development. She has extensive experience in drug discovery through early clinical development and was involved in the successful development, launch and/or post marketing support of several branded medicines including Seroqul™, Aloxi™, Dacogen™, Lucedra™.
At Johns Hopkins, Dr. Slusher leads the largest integrated drug discovery program on campus with a veteran team of medicinal chemists, assay developers, pharmacologists, toxicologists, and pharmacokinetics/drug metabolism experts with an average of 15 years Pharma R&D drug discovery experience. The team is engaged in identifying novel drug targets arising from the faculty’s research and translating them into new drug therapies for clinical development. She also founded and is leading the first International Consortium of Academic Drug Discovery (www.addconsortium.org) with over 150 university-led translational centers and 1500 members in an effort to coordinate and enhance academic drug discovery.

Jonathan Powell is a Professor of Oncology and Pharmacology at Johns Hopkins University School of Medicine. He received his AB from Dartmouth College and his M.D. Ph.D. from Emory University School of Medicine. His Post-graduate clinical training included the Osler Internal Medicine Residency Program at Johns Hopkins and Fellowship training in Hematology-Oncology at The Brigham and Women’s Hospital in Boston and NHLBI at the NIH. While at the NIH Dr. Powell was a post-doctoral fellow in the Laboratory of Dr. Ronald Schwartz. He joined the faculty of Johns Hopkins in 2001. Dr. Powell’s lab is interested in studying the cellular, biochemical and molecular mechanisms surrounding T cell activation, differentiation and tolerance. In elucidating fundamental insight into how the immune system is controlled, the lab has been investigating the role of mTOR and metabolism in immunology. This work established the principle that differential mTOR activation and subsequent metabolic pathway activation define T cell fate decisions. Overall, these studies have provided important insight into devising novel regimens for immunotherapy for cancer, bone marrow transplantation, autoimmune & inflammatory diseases. Dr. Powell is currently an Associate Director of the Bloomberg~Kimmel Institute for Cancer Immunotherapy and is the head of the program of Cancer and Immuno- metabolism.


Jonathan Powell is a Professor of Oncology and Pharmacology at Johns Hopkins University School of Medicine. He received his AB from Dartmouth College and his M.D. Ph.D. from Emory University School of Medicine. His Post-graduate clinical training included the Osler Internal Medicine Residency Program at Johns Hopkins and Fellowship training in Hematology-Oncology at The Brigham and Women’s Hospital in Boston and NHLBI at the NIH. While at the NIH Dr. Powell was a post-doctoral fellow in the Laboratory of Dr. Ronald Schwartz. He joined the faculty of Johns Hopkins in 2001. Dr. Powell’s lab is interested in studying the cellular, biochemical and molecular mechanisms surrounding T cell activation, differentiation and tolerance. In elucidating fundamental insight into how the immune system is controlled, the lab has been investigating the role of mTOR and metabolism in immunology. This work established the principle that differential mTOR activation and subsequent metabolic pathway activation define T cell fate decisions. Overall, these studies have provided important insight into devising novel regimens for immunotherapy for cancer, bone marrow transplantation, autoimmune & inflammatory diseases. Dr. Powell is currently an Associate Director of the Bloomberg~Kimmel Institute for Cancer Immunotherapy and is the head of the program of Cancer and Immuno- metabolism.


Barbara Slusher is Professor of Neurology (primary), Psychiatry, Neuroscience, Medicine and Oncology at Johns Hopkins School of Medicine and the Director of the Johns Hopkins Drug Discovery program (https://drugdiscovery.jhu.edu/). Before joining Johns Hopkins, she spent 18 years in the pharmaceutical industry, including several years at the level of Senior Vice President of Research and Translational Development. She has extensive experience in drug discovery through early clinical development and was involved in the successful development, launch and/or post marketing support of several branded medicines including Seroqul™, Aloxi™, Dacogen™, Lucedra™.
At Johns Hopkins, Dr. Slusher leads the largest integrated drug discovery program on campus with a veteran team of medicinal chemists, assay developers, pharmacologists, toxicologists, and pharmacokinetics/drug metabolism experts with an average of 15 years Pharma R&D drug discovery experience. The team is engaged in identifying novel drug targets arising from the faculty’s research and translating them into new drug therapies for clinical development. She also founded and is leading the first International Consortium of Academic Drug Discovery (www.addconsortium.org) with over 150 university-led translational centers and 1500 members in an effort to coordinate and enhance academic drug discovery.




Many cancer types are characterized as “glutamine-addicted” and targeting glutamine metabolism offers the potential for stopping tumor growth.
Operating at the intersection of cancer metabolism and immuno-oncology, our novel series of glutamine antagonists target anti-tumor growth and progression by:
Tumor-Directed, Broad Acting Glutamine Antagonist
This program was designed to preferentially deliver the active moiety to tumors:
Sirpiglenastat (DRP-104), our lead candidate, demonstrates significant advantages over the active moiety “DON”:
Glutamine is the most abundant amino acid in plasma and plays a critical role in cancer metabolism, feeding into the tricarboxylic acid cycle and being used for energy generation as well as providing building blocks for nucleotide, amino acid and lipid biosynthesis to support rapid proliferation. Multiple enzymes are involved in glutamine metabolism and as such, targeting one specific enzyme such as glutaminase may have a limited therapeutic impact. Broadly inhibiting other multiple glutamine-related pathways may potentially be more effective in shutting down tumor growth.
Glutamine is a nutrient used by cancer cells to increase proliferation and survival. In these rapidly dividing cells, glutamine is voraciously consumed as a source of energy, serves as nitrogen and carbon for biomass and is important in cell signaling. Numerous published reports have shown and highlight the breadth of glutamine-addicted tumors.
• Cell Metabolism (2012) 15(2): 157-170<br/> • Oncotarget (2015) 6(10): 7619-7631<br/> • Journal of Clinical Investigation (2015) 125(6): 2293-2306
• Nature Communications (2016) 7:11971</br> • International Journal of Clinical Experimental Pathology (2014) 7(3): 1093-1100
• International Journal of Clinical Experimental Pathology (2013): 7(1): 301-312</br> • Molecular Cancer Therapeutics (2014) 13(4): 890-901</br> • Oncogene (2016) 35(24): 3201-32018
• Journal of Proteome Research (2017) 16(3): 1315-1326
• PNAS (2015) 112(21): 6539-6544</br> • Journal of Clinical Investigation (2017) 127(5): 1631-1645
• Nature (2013) 496(7443): 101-105</br> • Histopathology (2015) 66(2): 234-243
• Cancer Biological Therapeutics (2012) 13(12): 1185-1194</br> • International Journal of Cancer (2015) 137(7): 1587-1597</br> • Oncotarget (2016) 7(32): 51462-51472</br> • Cell Reports (2017) 18(3): 601-610
• Neurochemistry International (2015) 88:6-9
Glutamine is a nutrient used by cancer cells to increase proliferation and survival. In these rapidly dividing cells, glutamine is voraciously consumed as a source of energy, serves as nitrogen and carbon for biomass and is important in cell signaling. Numerous published reports have shown and highlight the breadth of glutamine-addicted tumors.

Please refer to www.clinicaltrials.gov (Identifiers: NCT04471415 & NCT07249372) for additional clinical trial information.
We do not have an expanded access program available for DRP-104 at this time as we are currently in a first-in-human dose escalation phase to determine the safest maximal tolerated dose of DRP-104.
Targeting KEAP1-Mutant NSCLC With Broad-Acting Glutamine Antagonist
Oncology Times (2022) 44(13):1,12
Targeting Glutamine Metabolism Enhances Tumor Specific Immunity by Modulating Suppressive Myeloid Cells
Journal of Clinical Investigation (2020) 131859
Tumor Microenvironment, Metabolism, and Immunotherapy
New England Journal of Medicine (2020) 382:869-887
Glutamine Blockade Induces Divergent Metabolic Programs to Overcome Tumor Immune Evasion
Science (2019) 366(6468):1013-1021
Tumor-targeted Delivery of 6-diazo-5-oxo-L-norleucine (DON) using Substituted Acetylated Lysine Prodrugs
Journal of Medicinal Chemistry (2019) 62 (7): 3524–3538
We’re Not “DON” Yet: Optimal Dosing and Prodrug Delivery of 6-Diazo-5-oxo-L-norleucine
Molecular Cancer Therapeutics (2018) 17(9) 1824-1832
N-(Pivaloyloxy)alkoxy-carbonyl Prodrugs of the Glutamine Antagonist 6-Diazo-5-oxo-l-norleucine (DON) as a Potential Treatment for HIV Associated Neurocognitive Disorders
Journal of Medicinal Chemistry (2016) 60 (16): 7186–7198
AACR 2022
Elucidating the role of glutamine metabolism in head & neck squamous cell carcinoma
Michael Allevato, Daniela Nachmanson, Sally Trinh, Yumi Yokoyama, Olivier Harismendy, Robert Wild, and J. Silvio Gutkind
Download
ASCO 2021
Trial in progress abstract: Phase 1 & phase 2a, first-in-human study (FIH) of DRP-104, a broad glutamine antagonist, in adult patients with advanced solid tumors
Melissa Johnson, Deborah Doroshow, Tanguy Y. Seiwert, Michael K. Gibson, Vamsidhar Velcheti, Aaron Lisberg, Shetal A. Patel, Matthias Scheffler, Francois Lafleur, Margaret Dugan and Sunil Sharma
Download
AACR 2021
DRP-104, a broad acting glutamine antagonist, synergizes with immune checkpoint blockade in vivo
Yumi Yokoyama and Robert Wild
Download
Broad glutamine pathway inhibition by DRP-104 results in anti-tumor activity in hypermetabolic lung tumors resistant to PD-1 or osimertinib therapy
Morgan Brady, Milica Momcilovic, Chiara Montemurro, Yumi Yokoyama, Heather Christofk, Katerina Politi, Margaret Dugan, Aaron Lisberg, Robert Wild and David B. Shackelford
Download
Halting head and neck squamous cell carcinoma progression by broadly targeting glutamine metabolic pathways
Michael Allevato, Sally Trinh,Yumi Yokoyama, Robert Wild and J. Silvio Gutkind
Download
AACR 2020
Broad acting glutamine antagonism remodels the tumor microenvironment; induces distinctive immune modulation; and synergizes with immune checkpoint blockade
Yumi Yokoyama and Robert Wild
Download
Uncovering metabolic bottlenecks in KEAP1 mutant lung cancer
Sarah LeBoeuf, Shih Ming Huang, Christian Bahamon, Triantafyllia Karakousi, Warren Wu, Volkan Sayin, Robert Wild, and Thales Papagiannakopolous
Download
SITC 2019
DRP-104, a novel broad acting glutamine antagonist, induces distinctive immune modulation mechanisms and synergistic efficacy in combination with immune checkpoint blockade
Yumi Yokoyama, Michael Nedelcovych and Robert Wild
Download
DRP-104, a novel broad acting glutamine antagonist, induces durable anti-tumor responses in vivo by inhibiting tumor glutamine addiction, remodeling the tumor microenvironment and stimulating both the innate and adaptive immune system
Yumi Yokoyama, Michael Nedelcovych and Robert Wild
Download
AACR 2019
Tumor targeted delivery of glutamine antagonist: Use of CES1-/- Mice
Rana Rais, Jesse Alt, Ranjeet P. Dash, Alexandra J. Gadiano, Lukáš Tenora, Kateřina Novotná, Pavel Majer, Barbara S. Slusher
Download
Targeting glutamine metabolism disables warburg physiology by inhibiting proximal glycolysis and krebs cycle rewiring
Liang Zhao, Matthew Arwood, Wei Xu, Im-Hong Sun, Im-Meng Sun, MinHee Oh, Chirag Patel, Ryan Jou, Robert Leone, Jesse Alt, Rana Rais, Barbara Slusher, Jonathan D. Powell
Download
Targeting glutamine metabolism leads to terminal differentiation in acute myeloid leukemia (AML)
Robert D. Leone, Im-Meng Sun, Min-Hee Oh, Roshan Chikarmane, Radhika Viswanathan, Sanjeda Patwari, Matthew Arwood, Gabriel Ghiaur, Jonathan Powell
Download
Targeting glutamine metabolism as a mean of treating a murine model of ovarian cancer and ascites development
Min‐Hee Oh, Meghan Travers, Stephen Brown, Liang Zhao, Im‐Meng Sun, Im‐Hong Sun, Matthew Arwood, Wei Xu, Samuel Collins, Robert Leone, Eva Prchalova, Rana Rais, Barbara Slusher, Maureen Horton, Cynthia Zahnow, Jonathan Powell
Download
JHU395, a nervous tissue penetrant glutamine antagonist, restricts growth of malignant peripheral nerve sheath tumor with inhibition of nucleotide synthesis
Kathryn M. Lemberg, Liang Zhao, Ying Wu, Vijayabhaskar Veeravalli, Jesse Alt, Joanna Marie H. Aguilar, Lukáš Tenora, Michael Nedelcovych, Cory Brayton, Pavel Majer, Jaishri Blakeley, Rana Rais, and Barbara S. Slusher
Download
AACR 2018
Targeting glutamine metabolism as a means of enhancing antitumor T-cell responses
Robert Leone, Judson Englert, Im-Meng Sun, Min-Hee Oh, Jesse Alt, Im-Hong Sun, Ada J. Tam, Pavel Majer, Rana Rais, Barbara Slusher and Jonathan Powell
Glutamine metabolic inhibition synergizes with L-asparaginase in MYCN-amplified neuroblastoma
Micah J. Maxwell, Brad Poore, Allison Hanaford, Jesse Alt, Rana Rais, Barbara S. Slusher, Charles G. Eberhart and Eric H. Raabe
Download
Targeting glutamine metabolism enhances tumor specific immunity by inhibiting the generation and function of suppressive myeloid cells
Min-Hee Oh, Im-Hong Sun, Liang Zhao, Im-Meng Sun, Wei Xu, Chirag Patel, Robert Leone, Ada J. Tam, Judd Englert, Pavel Majer, Rana Rais, Barbara Slusher, Maureen R. Horton and Jonathan D. Powell
Download
Novel prodrugs of the glutamine antagonist 6-diazo-5-oxo-norleucine (DON) as treatment for malignant peripheral nerve sheath tumor
Kathryn Lemberg, Ying Wu, Jesse Alt, Liang Zhao, Alexandra J. Gadiano, Chabely Rodriguez, Rana Rais, Pavel Majer, Jaishri Blakeley and Barbara Slusher
Download
AACR 2017
Metabolism as checkpoint: Induction of anti-tumor immune response with the novel glutamine antagonist JHU-083
Robert D. Leone, Judson M. Englert, Min-Hee Oh, Chih-Hsien Cheng, Rana Rais, Barbara Slusher, Jonathan D. Powell
Download
AACR 2022
Sirpiglenastat (DRP-104), a novel broad acting glutamine antagonist, has therapeutic potential in targeting KEAP1-mutant lung cancer
Thales Papagiannakopoulos
Download
Festival of Biologics USA, World Immunotherapy 2022
Sirpiglenastat (DRP-104), a broad acting glutamine antagonist, metabolically reprograms glutamine addicted cancer cells and significantly remodels the tumor microenvironment leading to anti-tumor immune responses
Download
The Fibrolamellar Cancer Foundation (FCF) and Dracen Pharmaceuticals announce initiation of a phase I/II study of DRP-104 in combination with Durvalumab in patients with fibrolamellar carcinoma (FLC)
February 14, 2024
PDF Version
FCF establishes partnership with Dracen Pharmaceuticals
July 11, 2023
Visit FCF website
Dracen Pharmaceuticals Announces Oral Presentation at Festival of Biologics World Immunotherapy Congress 2022
March 18, 2022
PDF Version
Dracen Announces DRP-104 (sirpiglenastat) Presentation at ASCO 2021 Virtual Annual Meeting
June 3, 2021
PDF Version
Dracen Announces DRP-104 (sirpiglenastat) Presentations at AACR Virtual Annual Meeting
April 12, 2021
PDF Version
Dracen Announces Clinical Collaboration with Merck
March 8, 2021
PDF Version
Dracen Pharmaceutical’s DRP-104 Granted U.S. FDA Fast Track Designation for the treatment of Non-Small Cell Lung Cancer
October 27, 2020
PDF Version
Dracen Pharmaceuticals Announces the Initiation of First-in-human Study of DRP-104 in Adult Patients With Advanced Solid Tumors
September 25, 2020
PDF Version
Dracen announces two DRP-104 presentations at AACR
June 22, 2020
PDF Version
Dracen Pharmaceuticals announces DRP-104 presentations at the 2019 Society of Immunotherapy of Cancer (SITC) Meeting
November 8, 2019
PDF Version
Dracen Announces AACR Presentation
April 12, 2018
PDF Version
Dracen Closes Financing to Advance Immunometabolism Pipeline
March 22, 2018
PDF Version
Dracen Licenses Immunometabolism Platform
January 11, 2018
PDF Version
General Information
info@dracenpharma.com
Business Development Inquiries
businessdevelopment@dracenpharma.com
Job Openings:
The company does not currently have any job openings.
Dracen Pharmaceuticals, Inc. is aware of employment scams which make false use of our company logo to defraud job seekers. Please be aware that if you are approached with such a request on behalf of Dracen, do not provide any personal information or pay any fees as the recruitment is fraudulent. All legitimate company emails come from @dracenpharma.com. Dracen accepts no responsibility for any costs or charges incurred as a result of fraudulent activity.